Proposals are reviewed and prioritized for funding based on the following:
-
Educational need and plan to deliver the educational activity to the intended audience
-
Learning objectives that are achievable and align with the educational need
-
Experience in designing educational programs and assessing the effectiveness of the activity on learners
-
Requested budget
-
Accreditation and compliance with ACCME Standards for Integrity and Independence in Accredited Continuing Education
-
Alignment with Galderma’s educational focus areas
REQUIRED ELEMENTS FOR SUBMISSION
The following information is required to submit a request:
- Letter of Request
- Activity Description
-
Target Audience
-
Anticipated Number of Attendees
-
Delivery Format
-
Areas of Interest to Galderma
-
Activity Agenda
-
Needs Assessment
-
Proposed Learning Objectives
-
Outcomes Assessment and Measurement Plan
-
Grantor Attendee Policy (if live meeting)
-
Specific Purpose of Funding (if monetary support)
-
Detailed, Itemized Budget
-
Accreditation Certificate (if accredited)
-
Type & Amount of Support Requested
Please note that changes may occur to our requirements as we periodically update our process.
AGREEMENT
If the request is approved, a contract will be sent through the portal submission for execution between the activity provider, the joint provider (if applicable), and Galderma. Support is contingent upon a fully executed contract, and compliance with all terms and conditions. The contract must be fully executed prior to the start date of the activity or no support will be provided.
BUDGET
All requests must include an itemized budget that includes the following:
-
Granted funds may only be used for the purpose specified in the grant request. For multi-supported activities, the purpose of funding must be designated for the educational portions of the activity pertinent to Galderma.
-
All anticipated sources of income (to include registration fees and other expected sources of monetary support from third parties). Anticipated sources of income must not exceed the anticipated costs of the activity (requested Galderma support by itself, or in combination with other expected sources of monetary support from third parties).
We are committed to maintaining integrity in all of our professional relationships. We operate under the highest ethical standards and in full compliance with all applicable laws, regulations, industry codes, and guidelines. Approval of IME support is never related to or conditioned upon past or future prescriptions or purchases of Galderma products. Furthermore, we do not offer, or provide funding to encourage or to reward the prescription, purchase, ordering, or recommending of Galderma products.
Our grant review and approval process is in full accordance with the recommendations and the guidance of the Accreditation Council for Continuing Medical Education (ACCME), the US Food and Drug Administration (FDA), the Pharmaceutical Research and Manufacturers Association (PhRMA), the Office of Inspector General (OIG) Compliance Program Guidance for Pharmaceutical Manufacturers, the PhRMA Code on Interaction with Health Care Professionals, the AdvaMed Code of Ethics on Interactions with Health Care Providers, and National Physician Payment Transparency Program: Open Payments "Physician Payment Sunshine Act", as well as internal Galderma policies.
SUBMISSION CONFIDENTIALITY
By submitting materials for review, you agree that the information is not confidential, nor proprietary, and may be referred to a number of different persons at Galderma to determine the level of interest in supporting your request.
DISCLOSURE OF SUPPORT
The activity provider is required to identify Galderma by name only. Disclosure to learners must not include Galderma’s corporate or product logos, trade names, or product group messages.
GRANTOR ATTENDEE POLICY
For live activities, you are required to submit details regarding your organization’s grantor attendee policy. Should we provide IME grant support, we reserve the right to monitor the activity at our discretion. Prior to the start date of the activity, we will notify the activity provider if a Galderma representative will be monitoring the activity. Complimentary access should be given for the entire duration of the activity. For clarification, this is for observation purposes only; no continuing education credits will be requested by the Galderma-designated monitor. If we are not permitted to monitor the activity, these details should be communicated upfront in the initial request for IME grant support.
Types of Eligible Activities
Galderma accepts requests for monetary and/or in-kind product support for the following types of independent Medical Education (ME) Activities that may be accredited (CME) and non-accredited (non-CME):
- Live Meetings
- National/Regional Events
- Workshops
- Symposia
- Live Webcast
- Enduring Materials/Activities
- Digital Activities/Programs
- Grand Rounds
- Journal Publications/Supplements
- Performance Improvement Initiatives
- Patient Education Initiatives
- Residency Program Aesthetic Product Training
- Single-Supported & Multi-Supported Events/Programs
Products Available for In-Kind Product Support
The following aesthetic products are available for request through a ME grant:
- Dysport® 300 Unit vial
-
Restylane® Kysse 1 mL syringe
- Restylane® Defyne 1 mL syringe
- Restylane® Refyne 1 mL syringe
- Restylane® Lyft 1 mL syringe
- Restylane® Silk 1 mL syringe
- Restylane-L® 1 mL syringe
- Sculptra® Aesthetic 367.5 mg vial
Support That Will Not be Considered
Galderma will not consider requests in support of the following:
- Unrestricted educational grants
- Support or payments made directly to individual physicians or groups of physicians
- Travel, lodging, honoraria, registration fees, or personal expenses for non-teacher or non-author participants of a ME Activity
- Textbooks
- Journal clubs
- Support for activities that have already occurred
- Non-buffet meals or individual meals not made available to all ME Activity attendees
- Grants that are intended to directly and commercially promote Galderma products
- Recreational or entertainment activities associated with educational programs
- Staff development or training
- Capital equipment purchases or operating expenses
- Building or construction funding
- Support for political organizations or lobbying activity
- Support for programs about business management with topics such as "coding” or “office management"
- Requests that create a conflict of interest for Galderma
- Support for specific religious activities or beliefs
- Support for labor unions
- Support for fraternal, service, or veteran’s organizations
- Support for organizations that discriminate on the basis of race, color, creed, sex, national origin, sexual orientation, age, or veteran or disability statuses
- Activities that occur outside of the U.S.
- Charitable contributions
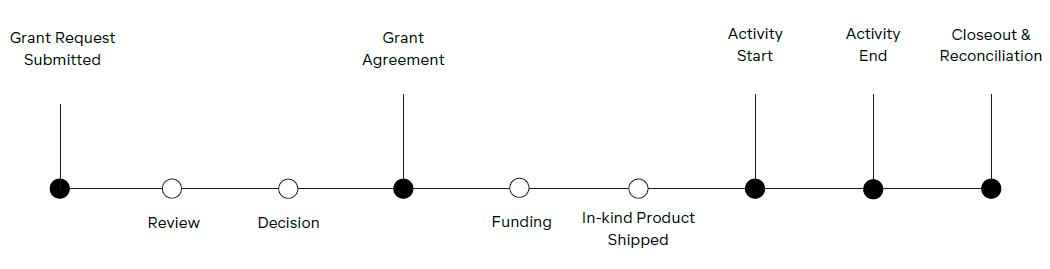
All Medical Education (ME) Grant Requests ("Grant Requests") must be submitted through Galderma's Medical Grants Portal ("Portal"). Grant Requests submitted through any other means will not be accepted. The steps of the submission process are further detailed below:
-
Submission: A Grant Request is submitted in the Portal by an Activity Provider.
-
Review: The Grant Request is routed to an internal committee for review and a written notification of the committee’s decision is sent.
-
Letter of Agreement: If the Grant Request is approved, a Letter of Agreement (LOA) is executed between Galderma and the Activity Provider before the start date of the ME Activity. Support from Galderma is contingent upon a fully executed LOA and compliance with the terms and conditions of the LOA.
-
Provision of Support: Once a LOA is executed, Galderma will provide support for the ME Activity and the ME Activity may start.
-
Payment: Payment in the form of an electronic funds transfer (EFT) will be made based on the terms established between Galderma and the Activity Provider.
-
Product: Product will be shipped directly to a licensed HCP authorized to receive product on behalf the Activity Provider. Shipping and delivery dates will be mutually agreed upon between Galderma and the Activity Provider.
-
Activity Completion: Upon completion of the Activity, the Activity Provider will furnish Galderma with a complete budget reconciliation and product reconciliation, as well as an Outcomes Measurement Report. For Activities that include multiple components, or occur over a longer period of time, progress reports and interim update notifications of any major progress (e.g., launch of program, interim outcomes data, completion of program, etc.) should also be provided to Galderma.
Please note that changes may occur as we periodically update our Process.
Submission Deadline
Galderma accepts Grant Requests on an ongoing basis. Grant Requests must be submitted at least 12 weeks in advance of the ME Activity’s start date to allow for internal review and processing.
Submission Confirmation
You will receive a notification email confirming the submission of each Grant Request.
Submission Status
You may log into the Portal at any time to check the current status of each submitted Grant Request.
Changes After Submission
Once a Grant Request is submitted, no changes can be made. However, if the internal committee requires clarification or has questions a "Request for Additional Information" will be issued.
Timing for Processing and Review
All Grant Requests must be submitted at least 12 weeks in advance of the planned ME Activity start so that Galderma is able to complete the review process and fully execute a Letter of Agreement prior to the ME Activity’s start date, if approved. Decisions may take longer if additional information is needed, or for larger educational programs, and additional processing time may also be needed around company and national holidays. Please note that Grant Requests may be declined due to insufficient processing time.
Request for Additional Information
A "Request for Additional Information" is sent when additional information is needed in order for the internal committee to make a determination regarding support. If the additional information provided is insufficient or not received in a timely manner, the Request may be denied.
Who May Apply
Medical Education (ME) Grant Requests (“Grant Requests”) must be submitted by an independent, reputable provider with a history of conducting scientific or educational programs in accordance with all applicable requirements and guidelines. “Independent” means that the provider must not be involved in advising or otherwise assisting Galderma with the conduct of its business, including the sale or marketing of its products.
The following types of organizations are eligible to submit ME Grant Requests:
- Medical Education Companies
- Hospitals
- Academic Institutions
- Community Health Centers
- Accredited and Non-accredited Providers
- Professional Societies and Associations
- Residency Programs
Galderma does not accept Grant Requests from or for individual physicians or groups of physicians.
Required Elements for Submission
The following information is required to submit a Grant Request:
- Letter of Request
- Activity Description
- Target Audience
- Anticipated Number of Attendees
- Delivery Format
- Areas of Interest to Galderma
- Activity Agenda
- Needs Assessment
- Proposed Learning Objectives
- Outcomes Assessment and Measurement Plan
- Inclusion of Moore’s Outcomes Levels
- Grantor Attendee Policy (if live meeting)
- Specific Purpose of Funding (if monetary support)
- Detailed, Itemized Budget
- Accreditation Certificate (if accredited)
- Type & Amount of Support Requested From Galderma
Please note that changes may occur to our requirements as we periodically update our process.
Budget
All Grant Requests must include an itemized budget with costs that reflect the expenses for the entire ME Activity to ensure that the amount of support provided is appropriate. Galderma will consider reasonable requests for funding in support of a ME Activity, but does not provide unrestricted educational grants. The following must be included in the itemized budget submitted with the Grant Request:
- The purpose of funding must be designated, and the designated purpose is the only one for which the funds may be used. For multi-supported activities, the purpose of funding must be designated for the educational portions of the ME Activity pertinent to Galderma.
- All anticipated sources of income (to include registration fees and other expected sources of monetary support from third parties) must be listed. All anticipated sources of income must not exceed the anticipated costs of the ME Activity (requested Galderma support by itself, or in combination with other expected sources of monetary support from third parties).
Letter of Agreement
Upon approval of a Grant Request, Galderma will execute a Letter of Agreement (LOA) with the Activity Provider. All ME Activities supported by Galderma must abide by the terms of Galderma’s LOA; no other LOAs will be accepted. Galderma may not provide any support to the Activity Provider until the LOA has been fully executed. The LOA must be executed before the start date of the ME Activity or no support will be provided.
The LOA will require the Activity Provider to:
- Retain control over the design and conduct of the Activity;
- Conduct the Activity independently, without any influence from Galderma;
- Ensure that the Activity is unbiased and objective and addresses all relevant treatment options, rather than focusing on a single product;
- Provide meaningful disclosure to the audience (i.e., disclosure that is reasonably calculated to reach the relevant audience in a manner that will alert them to potential biases) at the beginning of the Activity regarding:
- Galderma’s financial support;
- Any significant professional or financial relationships (i.e., relationships that may give rise to an actual or perceived conflict of interest) between Galderma and the individual presenters or moderators (e.g., investigator, grant recipient, etc.); and
- Whether unapproved uses of products will be discussed.
- Make available a reasonable opportunity for discussion and a question-and-answer session at the end of the Activity;
- Acknowledge Galderma as a source of funding for the Activity in any written materials; and
- Return any unused grant funds to Galderma.
Enduring Materials
Based on the information provided in the initial Grant Request, the LOA must indicate whether the Activity Provider will make the ME Activity available to learners through the production of Enduring Materials. Memorializing the Activity Provider’s intent to create Enduring Materials in the LOA ensures that Galderma’s decision to provide funding to support the creation of Enduring Materials is made without any knowledge of the ME Activity’s favorability to Galderma.
At the beginning of any Enduring Materials that are produced for a ME Activity, the Activity Provider must disclose the following information:
- The format of the Enduring Material;
- The start and end date of the Enduring Material; and
- How the Enduring Material will be updated, if applicable.
For Enduring Materials that are available to learners for a period longer than one (1) year, the Activity Provider will be required to update the Enduring Materials to reflect any new scientific developments. Enduring Materials must be reviewed at least once every three (3) years. Galderma may not be involved in providing or distributing ME Enduring Materials.
Updates
Activity Providers are required to notify Galderma of any of the following updates:
- Change of Scope; and
- Progress Reports, if applicable.
Post-Activity Reconciliations
Upon completion of the ME Activity, the Activity Provider must submit the following reconciliations:
- Outcomes Measurement Report;
- Budget Reconciliation; and
- Product Reconciliation.
If Galderma does not receive all reconciliations, the Activity Provider will not be allowed to request additional support from Galderma.
Change of Scope
Any ME Activity that undergoes significant changes requires a “Change of Scope” notification to Galderma. These changes will be re-reviewed by the committee and could result in a change in the support that Galderma has committed to that ME Activity. These changes must be submitted in writing to usmedicalgrants@galderma.com. Please submit the following information:
- Reference number of original ME Activity;
- Changes from the initial grant request;
- Rationale for changes; and
- Any updated documents (e.g., activity detail, agenda, brochure, budget, etc.).
Progress Reports
For ME Activities that include multiple components, or occur over a longer period of time, progress reports and interim update notifications of any major progress (e.g., launch of program, completion of program, etc.) should be provided to Galderma. Progress report timing will be dependent on the length of the ME Activity and may be monthly, bi-monthly, or quarterly.
Outcomes Measurement Report
The Outcomes Measurement Report should detail the following:
- Program Summary
- Type of Event/Activity
- Participation Demographics
- An overall summary that demonstrates the ME Activity met its stated learning objectives
- Participant Evaluation Form Summary
- Summary of any pre-test and post-test results
- Post-activity Results Summary
Budget Reconciliation
The Activity Provider must be able to produce accurate documentation detailing the receipt and expenditure of Galderma’s monetary grant support. This budget reconciliation must detail the following:
-
Planned expenses as provided in the initial grant request application;
-
Actual expenses spent during the course of the ME Activity, including how Galderma’s funds were allocated;
-
Unused support from Galderma; and
-
Sources of income for the Activity (multi-supported activities should list all sources of income, not just the granted funds from Galderma).
If there are any unused grant funds after ME Activity completion, the Activity Provider will be required to refund an amount proportional to the amount of funding Galderma originally provided.
Product Reconciliation
The Activity Provider will certify in writing that all product was used during the ME Activity, or that there is remaining unused or partially used product that needs to be returned or destroyed. The Activity Provider will send a notification e-mail following the ME Activity completion.
 Just a moment, the page is loading...
Just a moment, the page is loading...
 Just a moment, the page is loading...
Just a moment, the page is loading...